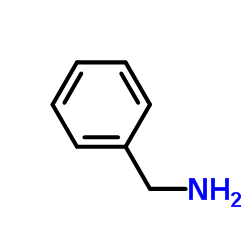
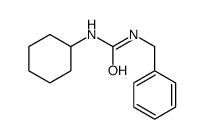
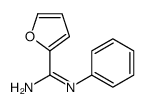
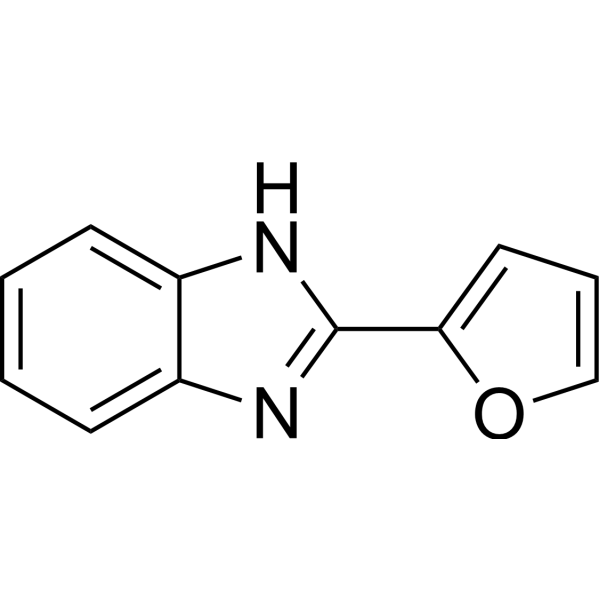
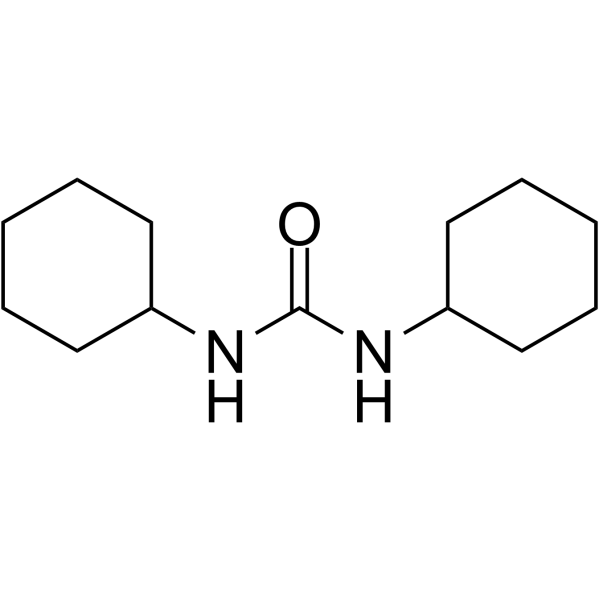
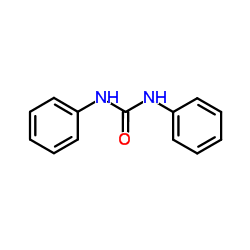
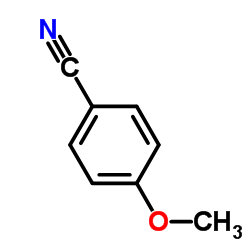
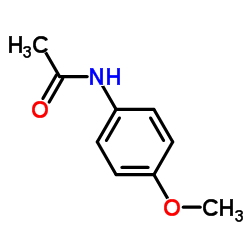
|
~% |
|
~63% |
|
~70% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |