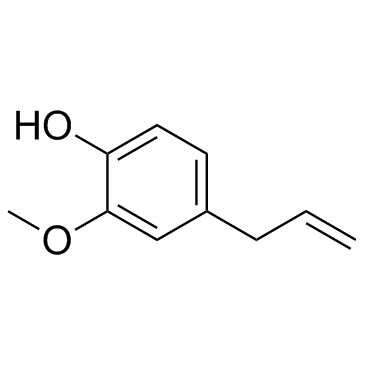
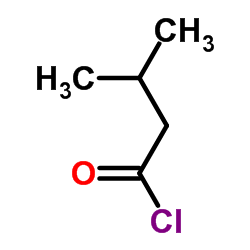
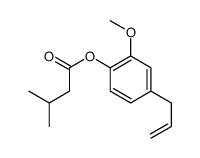
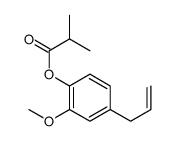
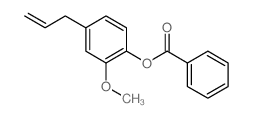
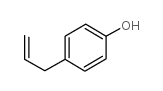
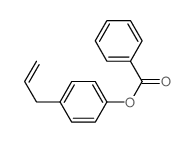
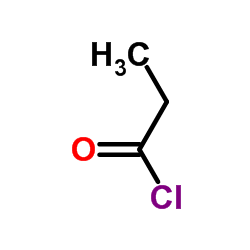
|
~88% |
|
~85% |
|
~77% |
|
~% |
|
~91% |
|
~69% |
|
~82% |