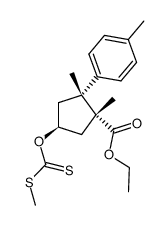
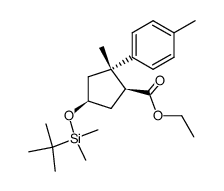
|
~98% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
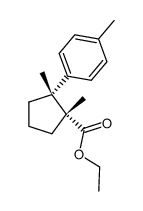
![[(1R,2R)-1,2-dimethyl-2-(4-methylphenyl)cyclopentyl]methanol结构式](https://image.chemsrc.com/caspic/151/740800-48-0.png)