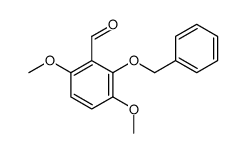
|
~94% |
|
~% |
|
~% |
|
~97% |
|
~% |
|
~% |

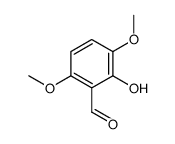
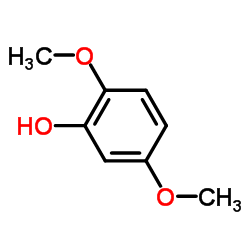
![[3,6-dimethoxy-2-(methoxymethoxy)phenyl]methanol结构式](https://image.chemsrc.com/caspic/202/211935-27-2.png)