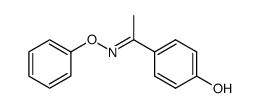
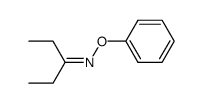
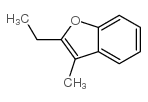
|
~82% |
|
~% |
|
~76% |
|
~93% |
|
~82% |
|
~86% |
|
~89% |

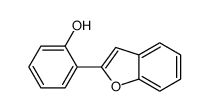

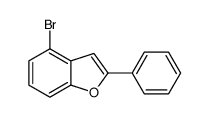

![6H‐benzofuro[3,2‐c][1benzopyran]结构式](https://image.chemsrc.com/caspic/160/239-33-8.png)
![6H-Benzofuro[3,2-c][1]benzopyran-6-one结构式](https://image.chemsrc.com/caspic/420/479-12-9.png)
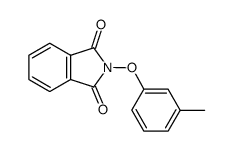
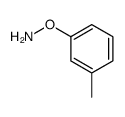
![5-硝基-2-苯基苯并[b]呋喃结构式](https://image.chemsrc.com/caspic/094/16237-97-1.png)