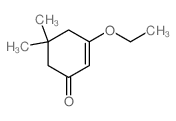
|
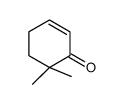
~0% |
|
~90% |
|
~% |
|
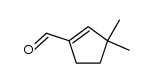
~81% |
|
~12% |
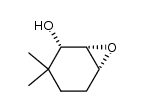

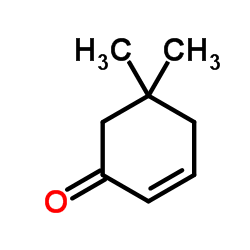
![3,3-dimethyl-7-oxabicyclo[4.1.0]heptan-5-one结构式](https://image.chemsrc.com/caspic/370/17421-93-1.png)