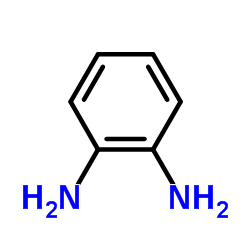
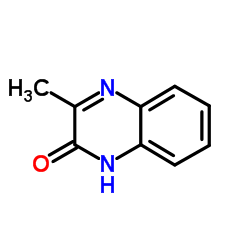
|
~% |
|
~% |
|
~83% |
|
~44% |
|
~44% |
![3-(N,N-Dimethylaminocarbonyl)-furo[2,3-b]quinoxaline hydrochloride结构式](https://image.chemsrc.com/caspic/377/73894-37-8.png)
![N-(2-aminophenyl)furo[3,2-b]quinoxaline-3-carboxamide结构式](https://image.chemsrc.com/caspic/202/93446-71-0.png)
![3-(N-2-hydroxyphenylcarbamoyl)furo[2,3-b]quinoxaline结构式](https://image.chemsrc.com/caspic/175/100633-77-0.png)