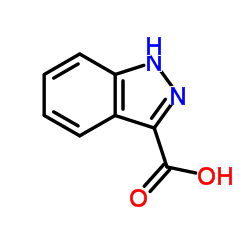
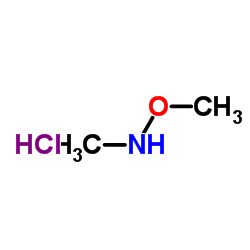
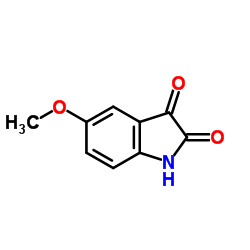
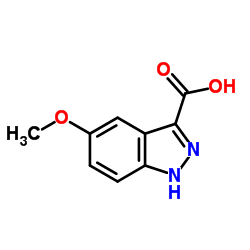
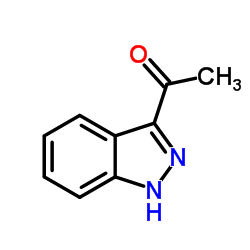
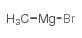|
~92% |
|
~87% |
|
~72% |
|
~% |
|
~% |
|
~% |
|
~84% |
|
~10% |
|
~82% |
|
~% |
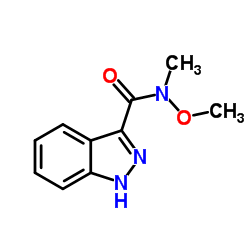


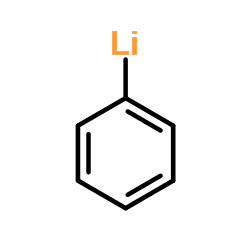
![tert-butyl 3-{[methoxy(methyl)amino]carbonyl}-1H-indazole-1-carboxylate结构式](https://image.chemsrc.com/caspic/402/887577-66-4.png)