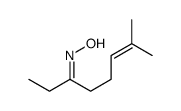
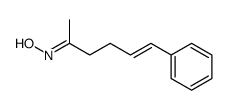
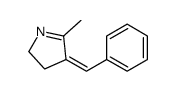
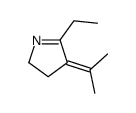
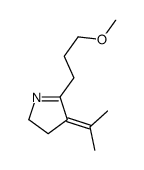
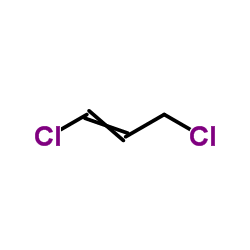
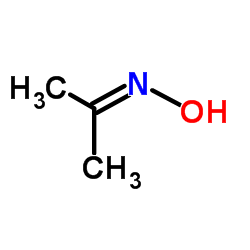
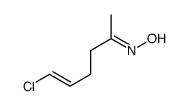
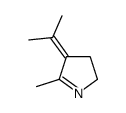
|
~% |
|
~% |
|
~% |
|
~% |
|
~57% |
|
~% |
|
~% |
|
~% |
|
~72% |
|
~% |
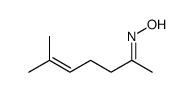
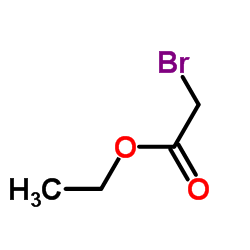
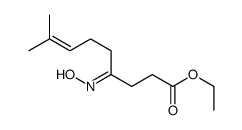
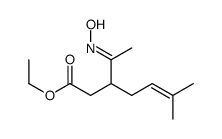
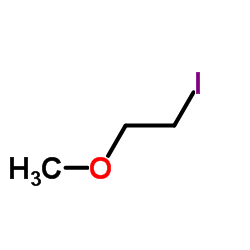
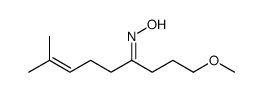
![N-[3-(2-methoxyethyl)-6-methylhept-5-en-2-ylidene]hydroxylamine结构式](https://image.chemsrc.com/caspic/271/89849-60-5.png)