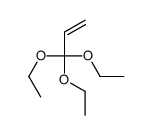
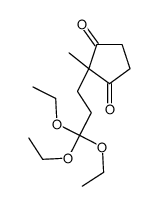
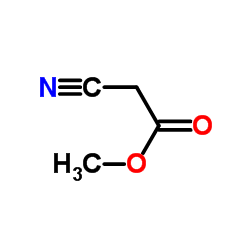
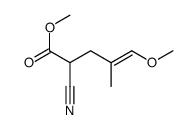
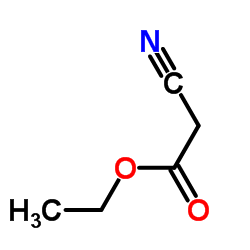
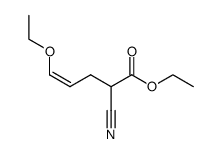
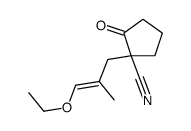
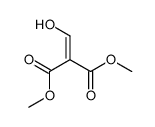
|
~67% |
|
~40% |
|
~26% |
|
~% |
|
~% |
|
~55% |
|
~54% |
|
~72% |
|
~52% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~76% |
|
~85% |
|
~% |
|
~51% |
|
~86% |
|
~% |
|
~68% |
|
~% |

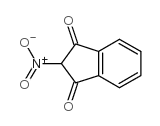
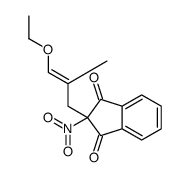
![BENZENE, [1-(DIETHOXYMETHYL)ETHENYL]结构式](https://image.chemsrc.com/caspic/293/80234-04-4.png)
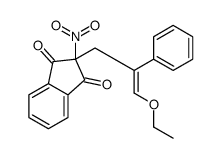
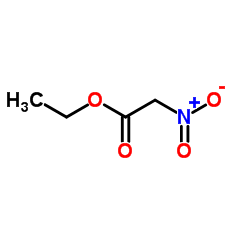
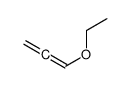

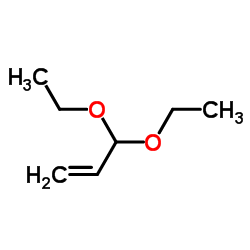
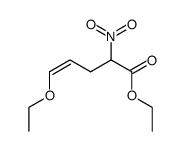
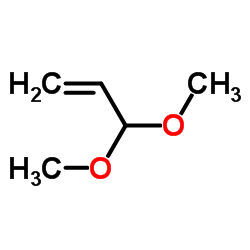
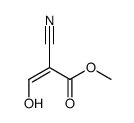
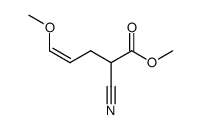
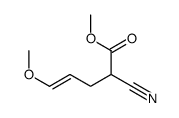
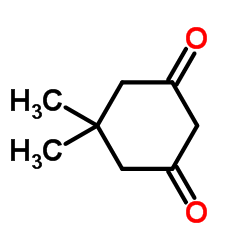
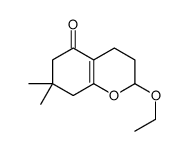
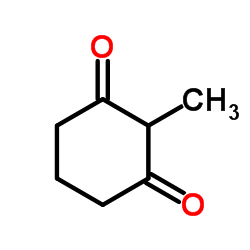

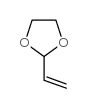
![2-[2-(1,3-dioxolan-2-yl)ethyl]-2-methylcyclohexane-1,3-dione结构式](https://image.chemsrc.com/caspic/159/87698-51-9.png)

![1-[2-(1,3-dioxolan-2-yl)ethyl]-2-oxocyclopentane-1-carbonitrile结构式](https://image.chemsrc.com/caspic/111/87698-52-0.png)
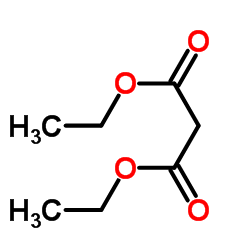
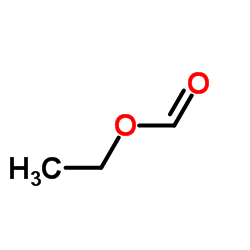
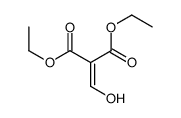
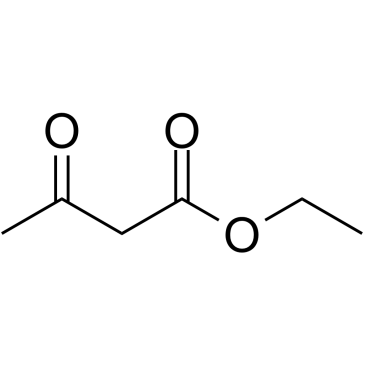

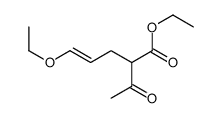
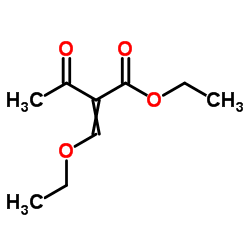

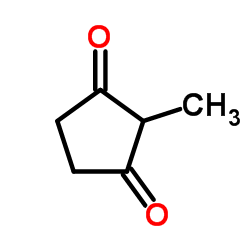
![2-[2-(1,3-dioxolan-2-yl)ethyl]-2-methylcyclopentane-1,3-dione结构式](https://image.chemsrc.com/caspic/280/87698-21-3.png)