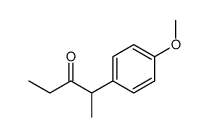
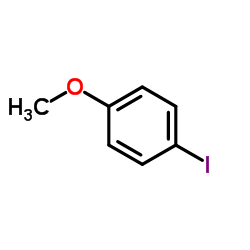
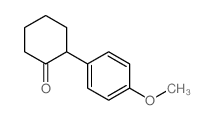
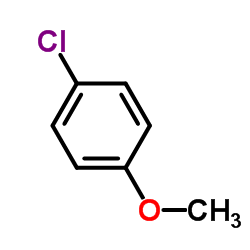
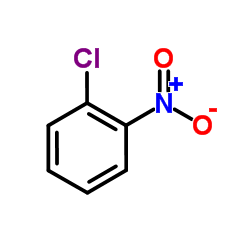
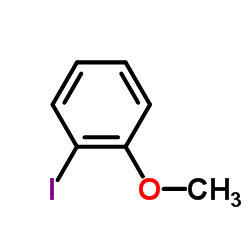
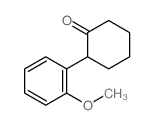
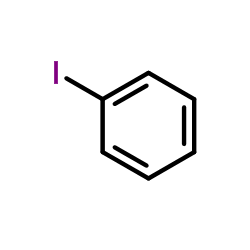
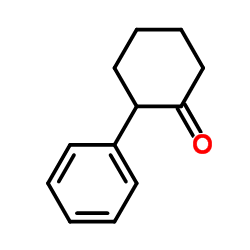
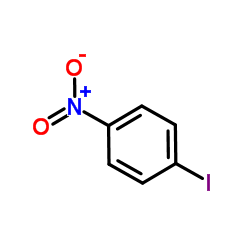
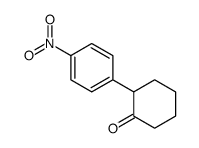
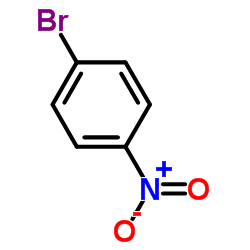
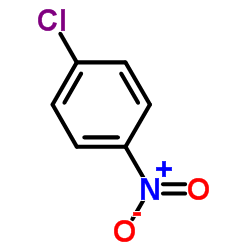
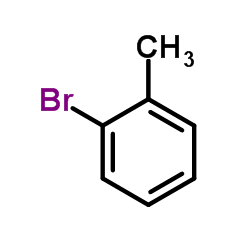
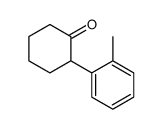
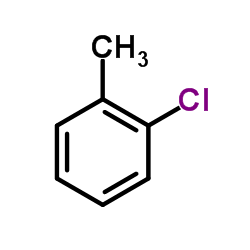
|
~70% |
|
~84% |
|
~65% |
|
~24% |
|
~72% |
|
~75% |
|
~67% |
|
~68% |
|
~61% |
|
~70% |
|
~55% |
![[(1-(ethyl)propenyl)oxy]trimethylsilane结构式](https://image.chemsrc.com/caspic/468/17510-47-3.png)