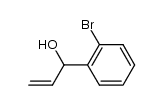
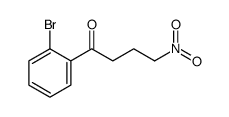
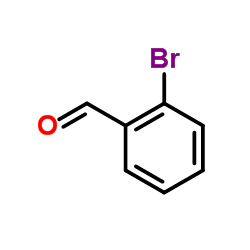
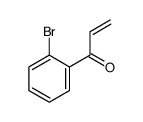
|
~83% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~74% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~81% |
|
~% |
|
~% |
|
~18% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~59% |
|
~% |

![2-[2-(2-bromophenyl)ethyl]-2-chlorocyclohexan-1-one结构式](https://image.chemsrc.com/caspic/115/823809-69-4.png)
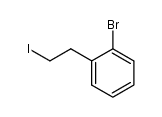
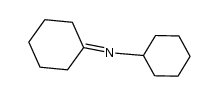
![2-[(2-bromophenyl)methyl]cyclohexan-1-one结构式](https://image.chemsrc.com/caspic/464/91911-21-6.png)
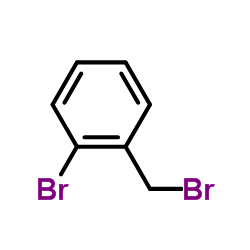
![2-[2-(2-bromophenyl)ethyl]cyclohex-2-en-1-one结构式](https://image.chemsrc.com/caspic/260/823809-70-7.png)

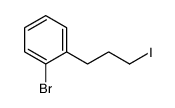

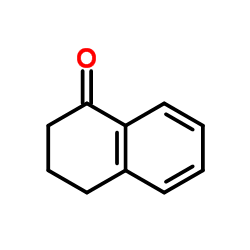
![2-[2-(2-bromo-5-methoxyphenyl)ethyl]cyclohexanone结构式](https://image.chemsrc.com/caspic/344/455330-80-0.png)
![2-bromo-2-[2-(2-bromo-5-methoxyphenyl)ethyl]cyclohexan-1-one结构式](https://image.chemsrc.com/caspic/098/823809-74-1.png)
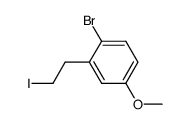
![2-[(2-bromophenyl)methyl]cyclopentan-1-one结构式](https://image.chemsrc.com/caspic/238/132452-24-5.png)