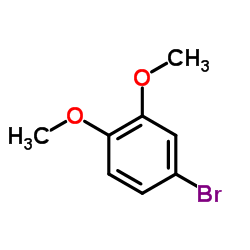
|
~69% |
|
~% |
|
~72% |
|
~56% |
|
~% |
|
~% |
|
~% |
|
~39% |
|
~% |
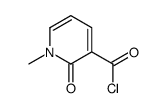


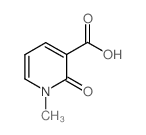
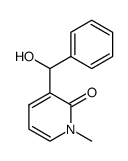
![5-(3,4-dimethoxyphenyl)benzo[c][2,7]naphthyridin-4(3H)-one结构式](https://image.chemsrc.com/caspic/352/81755-06-8.png)
![5-(3,4-dimethoxyphenyl)-6-hydroxybenzo[f][2,7]naphthyridin-4-one结构式](https://image.chemsrc.com/caspic/132/81751-09-9.png)