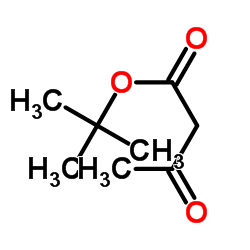
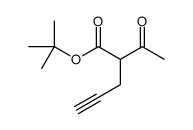
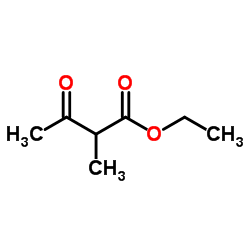
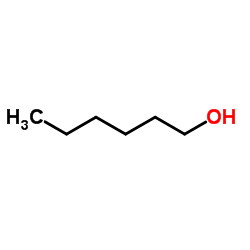
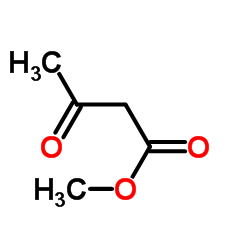
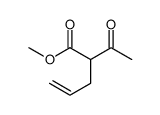
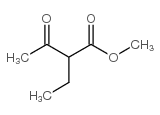
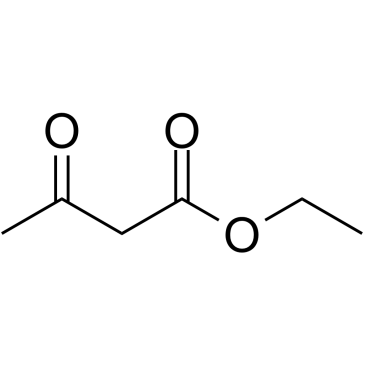
|
~20% |
|
~82% |
|
~2% |
|
~45% |
|
~75% |
|
~42% |