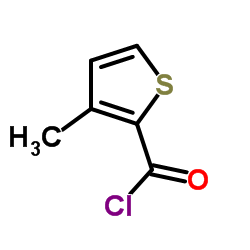
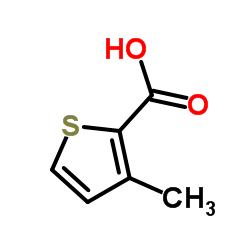
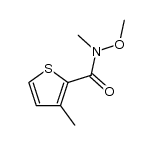
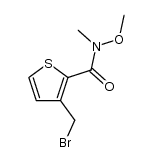
|
~79% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

![4-ethylsulfanyl-6-phenylthieno[2,3-c]furan结构式](https://image.chemsrc.com/caspic/343/181868-55-3.png)