|
~99% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~5% |
|
~% |
|
~0% |
|
~79% |
|
~% |
|
~81% |
|
~% |
|
~% |
|
~93% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~33% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~12% |
|
~% |
|
~90% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
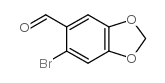

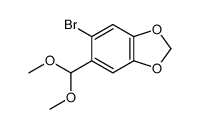
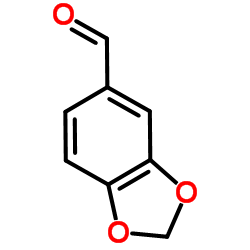
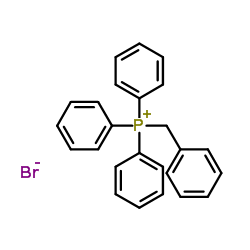
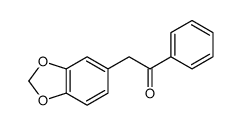

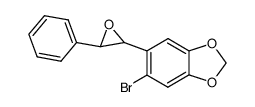
![Cyclobuta[f]-1,3-benzodioxol-5-ol, 5,6-dihydro-5-phenyl结构式](https://image.chemsrc.com/caspic/118/134454-85-6.png)
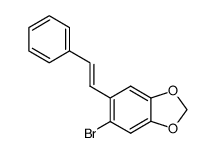
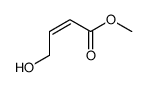




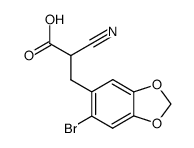
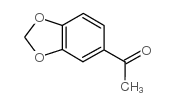
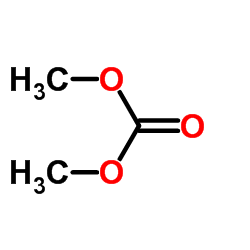
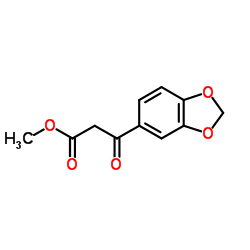
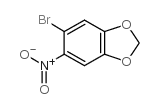
![5-BROMO-6-IODOBENZO[D][1,3]DIOXOLE结构式](https://image.chemsrc.com/caspic/060/94670-76-5.png)
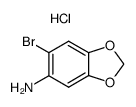
![5-溴-6-氟苯并[d][1,3]二氧戊环结构式](https://image.chemsrc.com/caspic/098/94670-75-4.png)
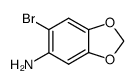
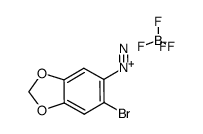

![5,8-Epoxynaphtho[2,3-d]-1,3-dioxole, 5,8-dihydro结构式](https://image.chemsrc.com/caspic/022/94670-77-6.png)