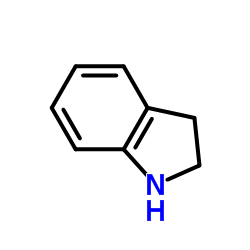
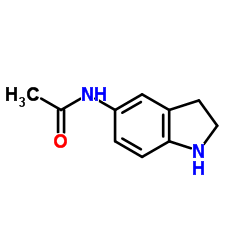
|
~92% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![1-(5-acetylpyrrolo[2,3-f]indol-1(5H)-yl)-2,2,2-trifluoroethan-1-one结构式](https://image.chemsrc.com/caspic/367/137601-30-0.png)
![1,5-dihydropyrrolo[2,3-f]indole结构式](https://image.chemsrc.com/caspic/128/7075-68-5.png)