|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
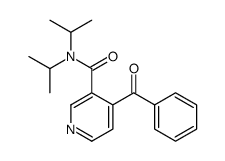

![1-phenyl-1,3-dihydrofuro[3,4-c]pyridin-3-one结构式](https://image.chemsrc.com/caspic/264/849322-39-0.png)
![1-chloro-1-phenylfuro[3,4-c]pyridin-3(1H)-one结构式](https://image.chemsrc.com/caspic/469/104867-64-3.png)
![1-hydroxy-1-phenyl-2H-pyrrolo[3,4-c]pyridin-3-one结构式](https://image.chemsrc.com/caspic/409/849322-40-3.png)
![4-[hydroxy(phenyl)methyl]-N,N-diisopropylnicotinamide结构式](https://image.chemsrc.com/caspic/423/104084-46-0.png)