|
~35% |
|
~% |
|
~50% |
|
~% |
|
~85% |
|
~% |
|
~96% |
|
~89% |
|
~98% |
|
~75% |
|
~% |
|
~73% |
|
~97% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~88% |
|
~54% |
|
~74% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~84% |
|
~% |
|
~75% |
|
~97% |
|
~87% |
|
~% |
|
~% |
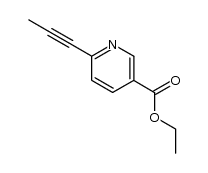
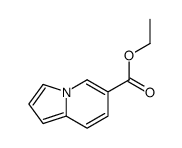
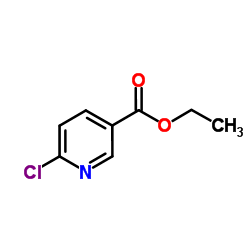

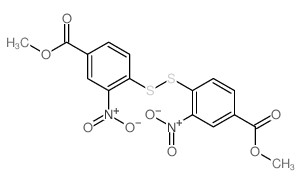
![苯并[d]噻唑-5-羧酸甲酯结构式](https://image.chemsrc.com/caspic/198/478169-65-2.png)
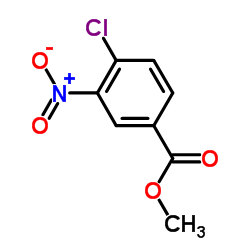
![Carbamic acid, (1R,3R,4S)-1-azabicyclo[2.2.1]hept-3-yl-, 1,1-dimethylethyl结构式](https://image.chemsrc.com/caspic/337/688790-05-8.png)
![(3S, 4R)-1-AZA-BICYCLO[2.2.1]HEPT-3-YLAMINE结构式](https://image.chemsrc.com/caspic/343/508209-67-4.png)

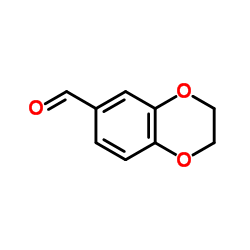
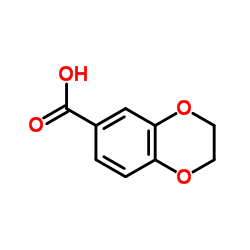
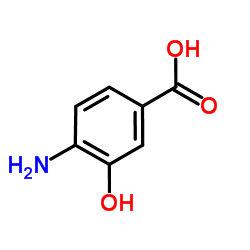


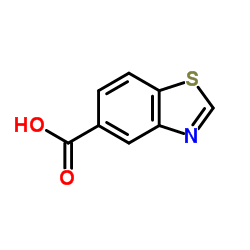
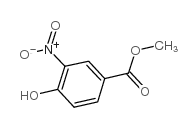
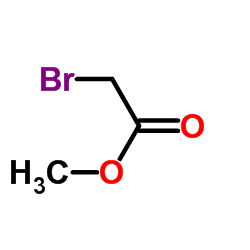
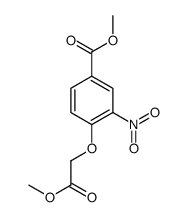
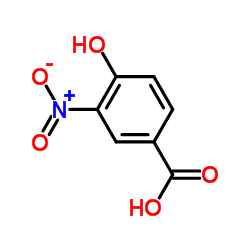


![3,4-二氢-2H-苯并[b][1,4]恶嗪-7-羧酸结构式](https://image.chemsrc.com/caspic/042/851202-96-5.png)
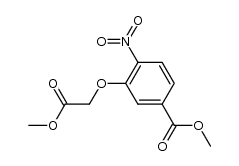

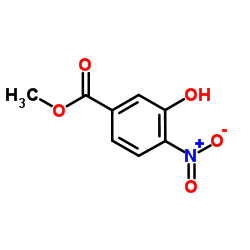

![3,4-二氢-2H-苯并[1,4]恶嗪-6-甲酸甲酯结构式](https://image.chemsrc.com/caspic/301/758684-29-6.png)
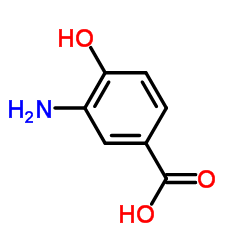
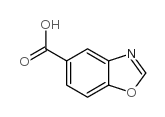
![3,4-二氢-2H-苯并[1,4]噁嗪-6-羧酸结构式](https://image.chemsrc.com/caspic/467/918789-44-3.png)

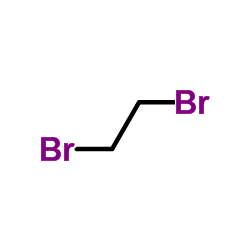
![2,3-二氢-[1,4]二噁英[2,3-c]吡啶-7-羧酸甲酯结构式](https://image.chemsrc.com/caspic/229/527681-12-5.png)
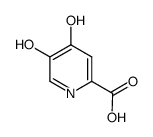

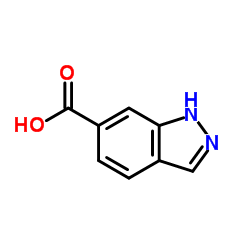
![2,3-二氢-1,4-二噁英并[2,3-c]吡啶-7-羧酸结构式](https://image.chemsrc.com/caspic/191/527681-13-6.png)