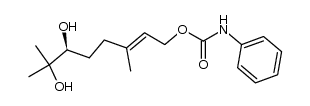
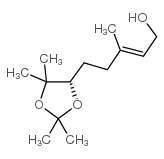
|
~78% |
|
~% |
|
~% |
|
~80% |
|
~% |
|
~% |

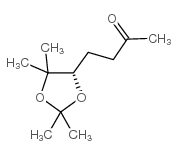
![[(2E)-3,7-dimethylocta-2,6-dienyl] N-phenylcarbamate结构式](https://image.chemsrc.com/caspic/431/57706-89-5.png)