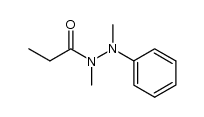
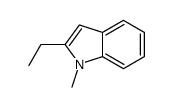
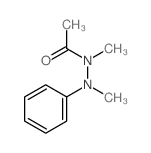
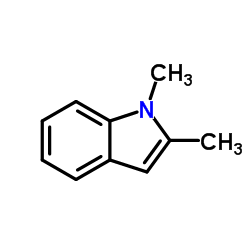
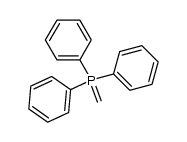
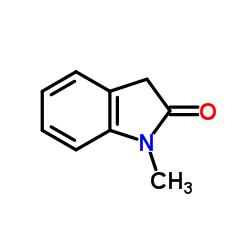
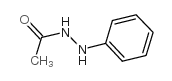
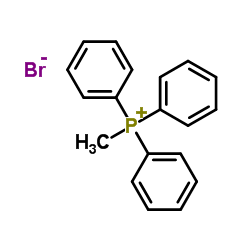
|
~46% |
|
~51% |
|
~76% |
|
~58% |
|
~87% |
|
~92% |
|
~74% |
|
~47% |