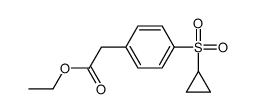
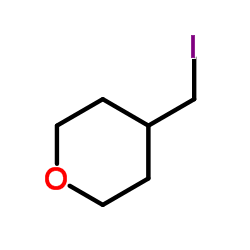
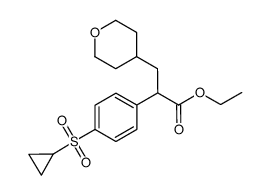
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~87% |
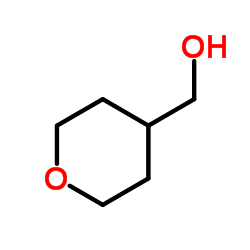
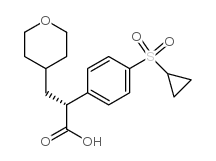

![ethyl [4-(cyclopropylthio)phenyl]acetate结构式](https://image.chemsrc.com/caspic/161/1058167-39-7.png)