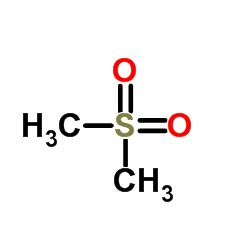
|
~67% |
|
~% |
|
~% |
|
~80% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~92% |
|
~0% |
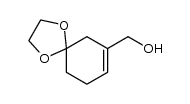
![1,4-dioxaspiro[4.5]dec-7-ene-7-carbaldehyde结构式](https://image.chemsrc.com/caspic/465/63517-54-4.png)

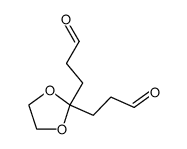

![Naphtho[2,3-b]furan-2(3H)-one,decahydro-7-hydroxy-8a-methyl-3,5-bis(methylene)-, (3aR,4aS,7S,8aR,9aR)结构式](https://image.chemsrc.com/caspic/062/5938-03-4.png)

![(3aR,4aS,7S,8aS,9aR)-7-((tert-butyldimethylsilyl)oxy)-3-(hydroxymethyl)-8a-methyl-5-methylenedecahydronaphtho[2,3-b]furan-2(3H)-one结构式](https://image.chemsrc.com/caspic/116/123966-86-9.png)

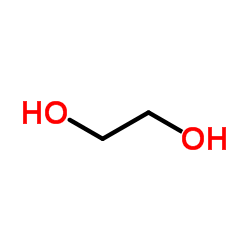
![methyl 1,4-dioxaspiro[4.5]dec-7-ene-7-carboxylate结构式](https://image.chemsrc.com/caspic/383/79678-14-1.png)