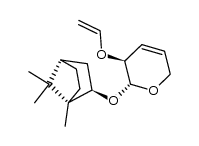
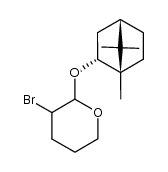
|
~87% |
|
~% |
|
~95% |
|
~95% |
|
~% |
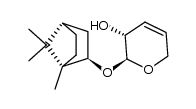
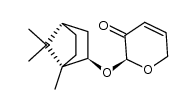
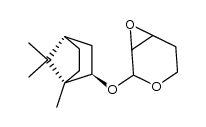
![[2(R)-(1-bornyloxy)-5,6-dihydro-2H-pyran-5(R)-yl]acetaldehyde结构式](https://image.chemsrc.com/caspic/046/100703-53-5.png)
![[2(R)-(1-bornyloxy)-5,6-dihydro-2H-pyran-5(R)-yl]acetic acid结构式](https://image.chemsrc.com/caspic/008/100703-54-6.png)