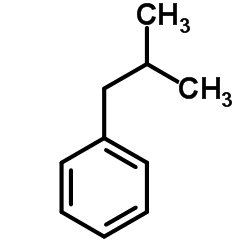
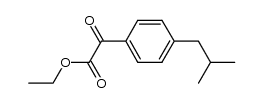
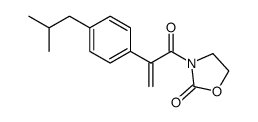
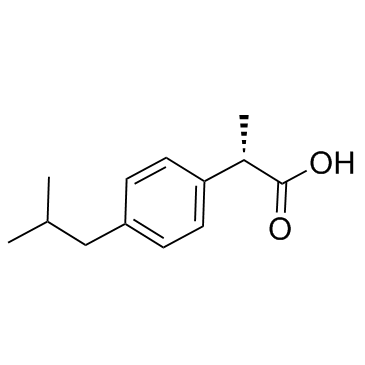
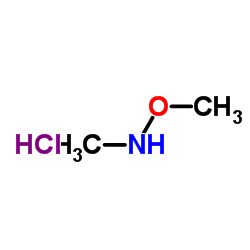
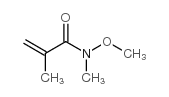
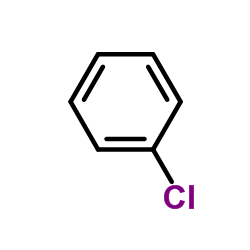
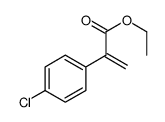
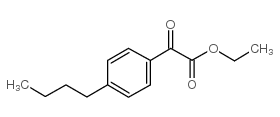
|
~70% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~79% |
|
~% |
|
~% |

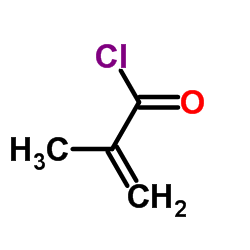
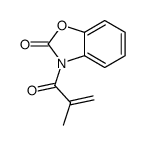
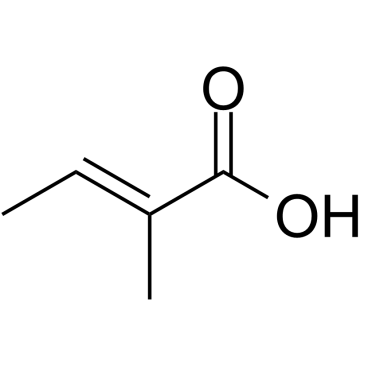

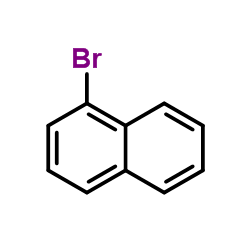
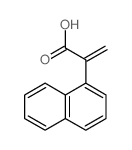

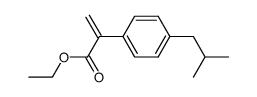
![N-[3-[(3-propoxybenzoyl)carbamothioylamino]phenyl]furan-2-carboxamide结构式](https://image.chemsrc.com/caspic/447/6448-14-2.png)