|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
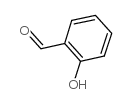
![[2-[(4-bromophenyl)iminomethyl]phenyl] 4-methoxybenzoate结构式](https://image.chemsrc.com/caspic/162/827308-19-0.png)
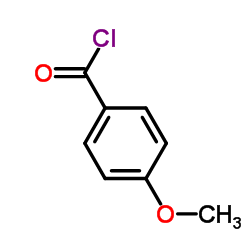

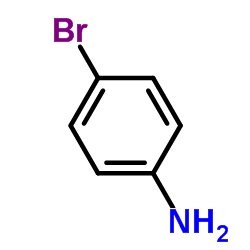
![[2-(phenyliminomethyl)phenyl] 4-methoxybenzoate结构式](https://image.chemsrc.com/caspic/473/479354-16-0.png)
