|
~79% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~46% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~61% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~84% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

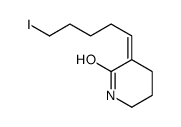

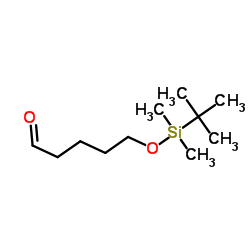


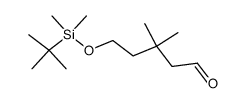
![6-[tert-butyl(dimethyl)silyl]oxyhexanal结构式](https://image.chemsrc.com/caspic/268/118794-69-7.png)
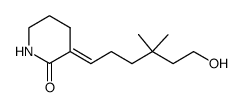
![6-{[tert-butyl(dimethyl)silyl]oxy}-4,4-dimethylhexanal结构式](https://image.chemsrc.com/caspic/079/911369-52-3.png)
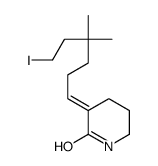
![(E)-3-(6-{[tert-butyl(dimethyl)silyl]oxy}-4,4-dimethylhexylidene)piperidin-2-one结构式](https://image.chemsrc.com/caspic/389/911369-57-8.png)