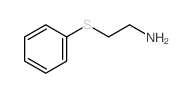
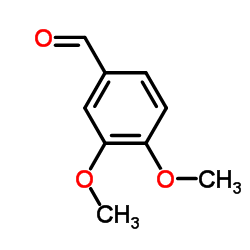
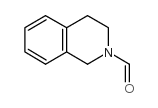
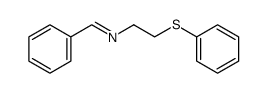
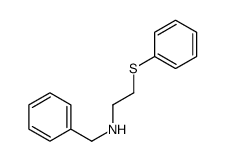
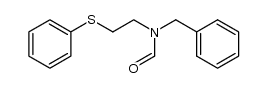
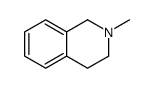
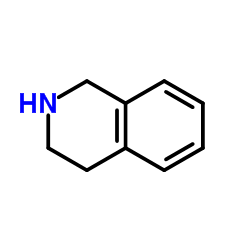
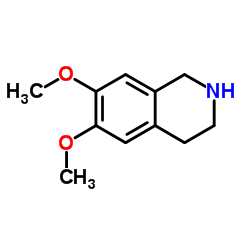
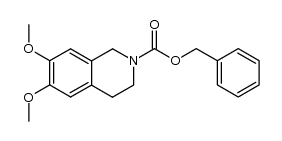
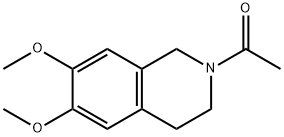
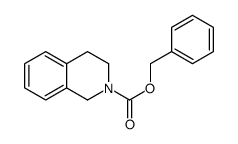
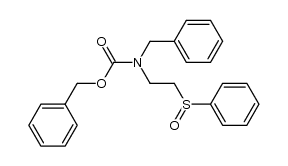
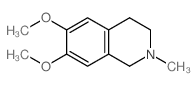
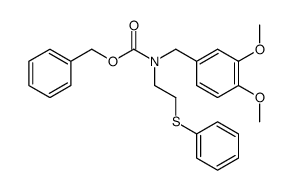
|
~% |
|
~% |
|
~% |
|
~71% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~91% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~95% |
|
~99% |
|
~89% |
|
~78% |
|
~58% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~97% |
|
~85% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

![N-[2-(benzenesulfinyl)ethyl]-N-[(3,4-dimethoxyphenyl)methyl]acetamide结构式](https://image.chemsrc.com/caspic/138/148679-46-3.png)