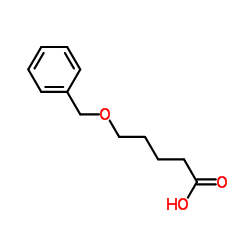
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
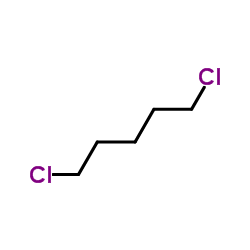

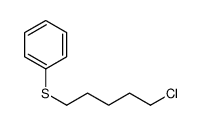

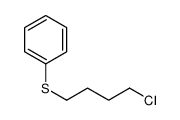

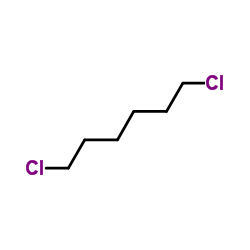

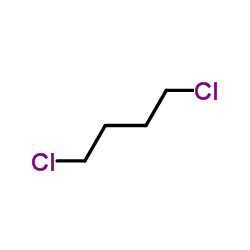
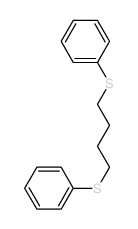

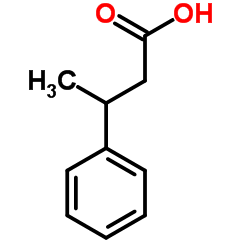


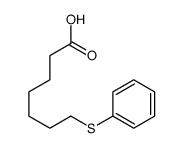

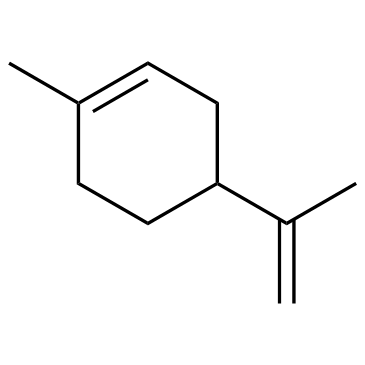
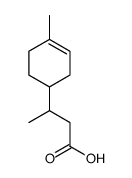

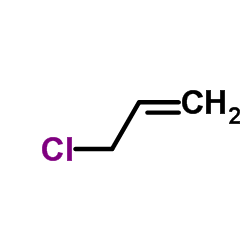
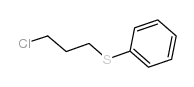
![Benzene,[(3-phenylpropyl)thio]结构式](https://image.chemsrc.com/caspic/352/30134-12-4.png)