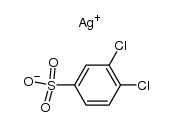
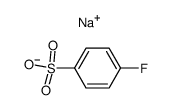
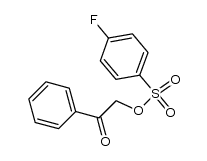
|
~% |
|
~% |
|
~97% |
|
~% |
|
~% |
|
~85% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |

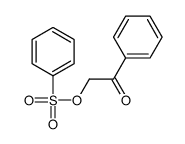
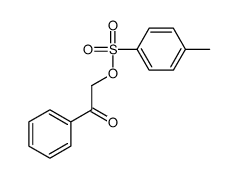
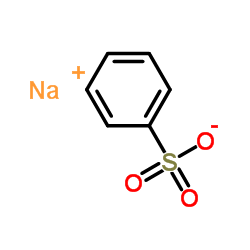
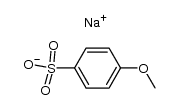



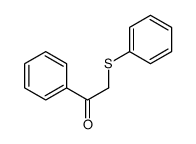
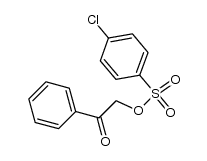
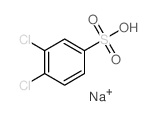
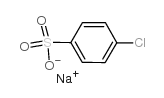
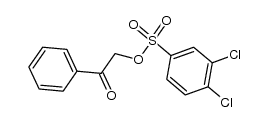
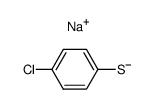
![Ethanone,2-[(4-chlorophenyl)thio]-1-phenyl结构式](https://image.chemsrc.com/caspic/245/30168-33-3.png)