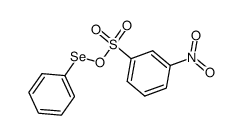
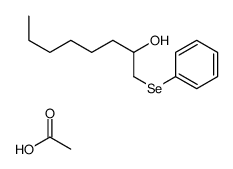
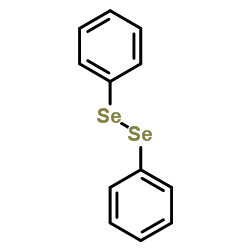
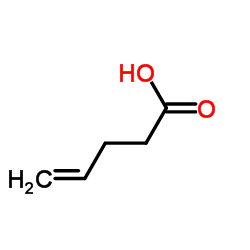
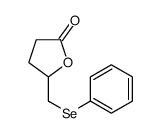
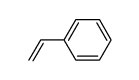
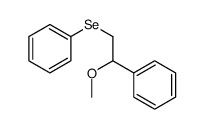
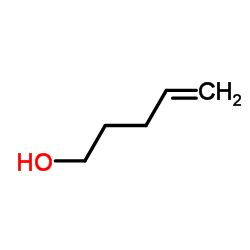
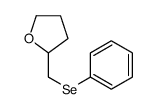
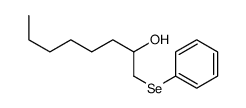
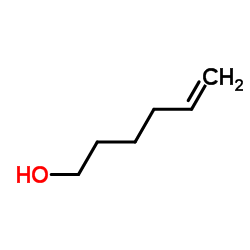
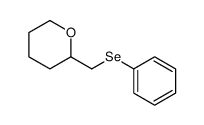
|
~71% |
|
~% |
|
~94% |
|
~% |
|
~10% |
|
~% |
|
~99% |
|
~% |
|
~43%
详细
|
|
~% |
|
~99% |