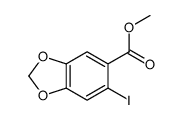
|
~% |
|
~99% |
|
~96% |
|
~% |
|
~% |
|
~% |
|
~98% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
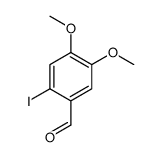
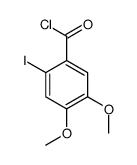
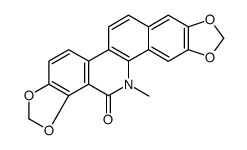
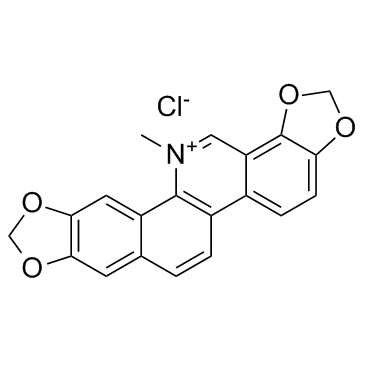

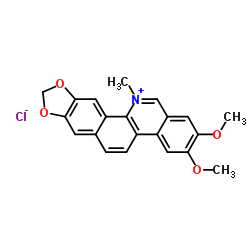



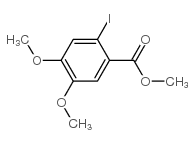
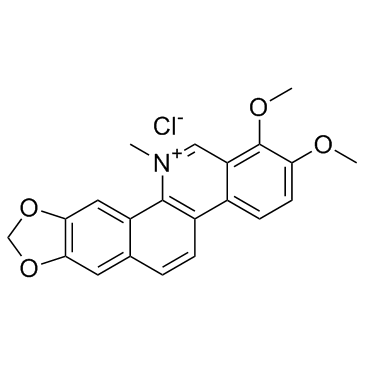
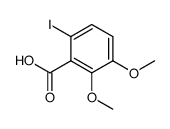
![6-iodobenzo[d][1,3]dioxole-5-carboxylic acid结构式](https://image.chemsrc.com/caspic/046/60229-66-5.png)