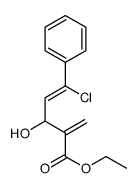
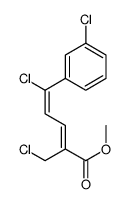
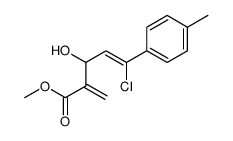
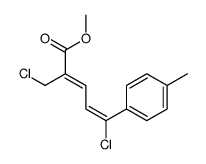
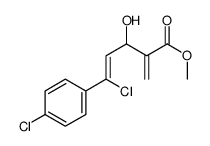
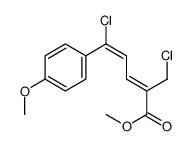
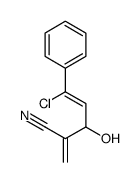
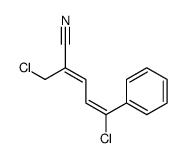
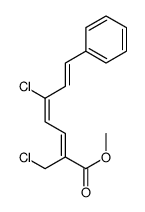
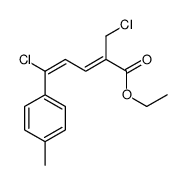
|
~91% |
|
~90% |
|
~93% |
|
~91% |
|
~90% |
|
~83% |
|
~88% |
|
~79% |
|
~88% |