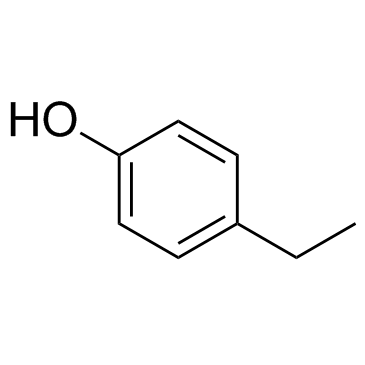
|
~% |
|
~% |
|
~92% |
|
~99% |
|
~82% |
|
~% |
|
~% |
|
~% |
|
~72% |
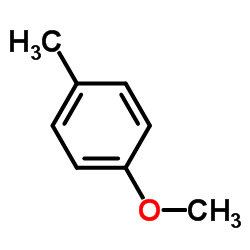
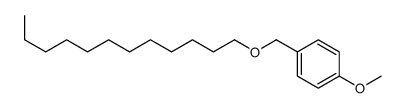
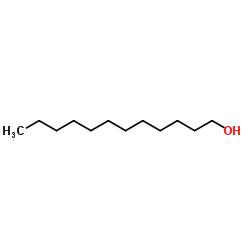
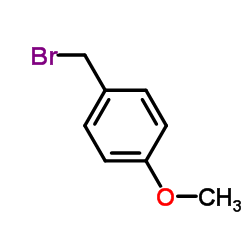

![(1S,2R,4S)-2-((4-methoxybenzyl)oxy)-1,7,7-trimethylbicyclo[2.2.1]heptane结构式](https://image.chemsrc.com/caspic/121/54384-77-9.png)

![Benzene,1-methoxy-4-[(4-nitrophenoxy)methyl]结构式](https://image.chemsrc.com/caspic/325/31574-13-7.png)

![1-[4-[(4-methoxyphenyl)methoxy]phenyl]ethanone结构式](https://image.chemsrc.com/caspic/010/72293-97-1.png)