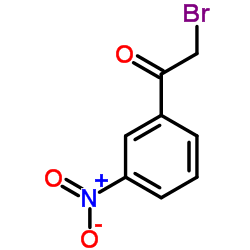
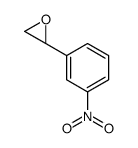
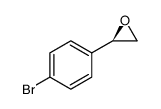
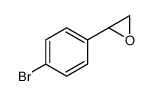
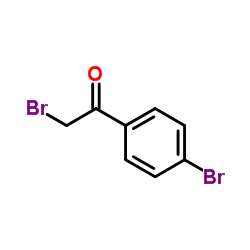
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
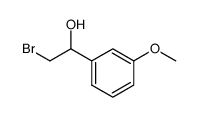

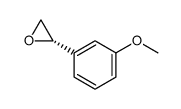
![3'-甲氧基苯乙酰溴[用于高效液相色谱标记]结构式](https://image.chemsrc.com/caspic/447/5000-65-7.png)