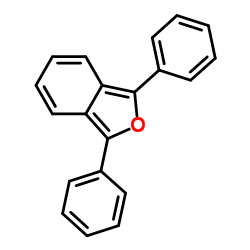
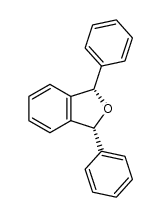
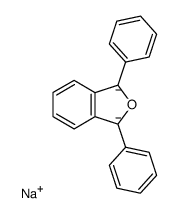
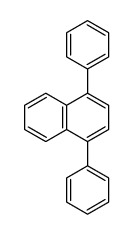
|
~37% |
|
~% |
|
~95% |
|
~% |
|
~% |