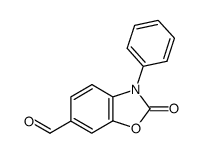
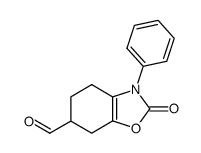
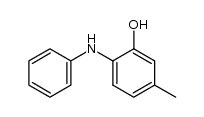
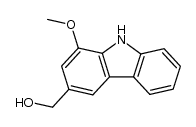
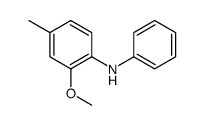
|
~73% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
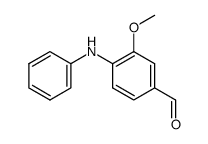
![2-二苯基甲基-2-氮杂螺[3.3]-5-庚酮结构式](https://image.chemsrc.com/caspic/245/723-97-7.png)