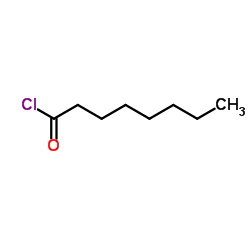
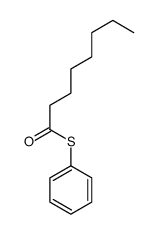
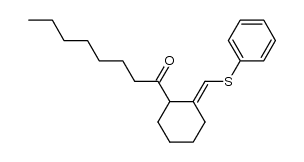
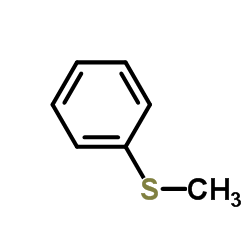
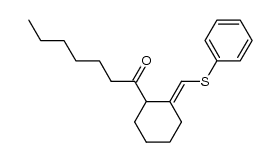
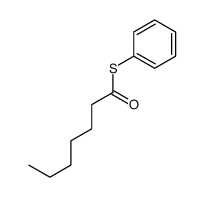
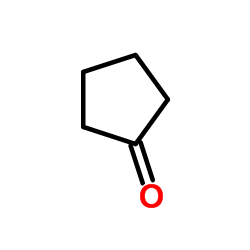
|
~94% |
|
~% |
|
~9% |
|
~% |
|
~9% |
|
~% |
|
~86% |
|
~93% |

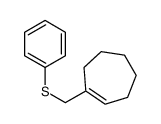
![[cyclohexen-1-yl(phenylsulfanyl)methyl]-trimethylsilane结构式](https://image.chemsrc.com/caspic/459/100693-33-2.png)