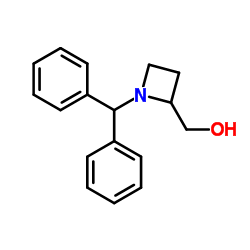
|
~45% |
|
~% |
|
~80% |
|
~38% |
|
~% |
|
~99% |
|
~% |
|
~% |
|
~% |
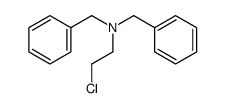
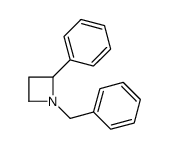
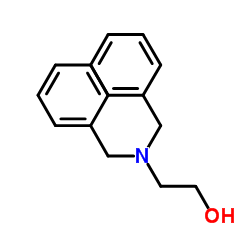

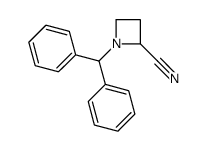

![2-[(diphenylmethyl)amino]Ethanol结构式](https://www.chemsrc.com/extcaspic/430/18809-63-7.png)