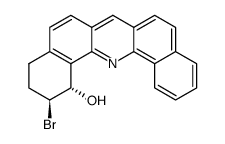
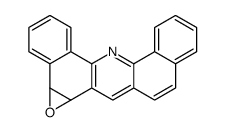
|
~10% |
|
~1% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~92% |
|
~% |
|
~% |
|
~% |
![(1S,2S)-2-bromo-1,2,3,4-tetrahydrodibenzo[c,h]acridin-1-yl 3,3,3-trifluoro-2-methoxy-2-phenylpropanoate结构式](https://image.chemsrc.com/caspic/357/93716-18-8.png)
![二苯并[c,h] ac啶结构式](https://image.chemsrc.com/caspic/301/224-53-3.png)
![1a,2,3,13c-tetrahydrobenzo[c]oxireno[2',3':5,6]benzo[1,2-h]acridine结构式](https://image.chemsrc.com/caspic/397/93781-05-6.png)

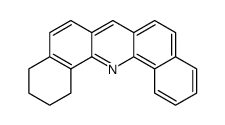
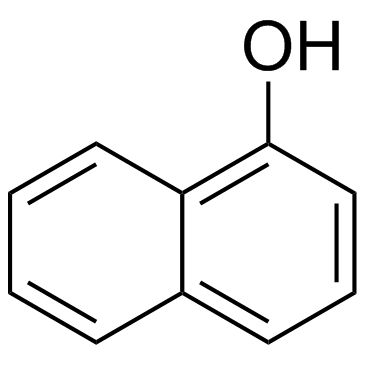

![1-hydroxy-1,2,3,4-tetrahydro-dibenz[c,h]acridine结构式](https://image.chemsrc.com/caspic/174/76186-80-6.png)