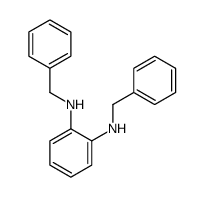
|
~20% |
|
~76% |
|
~74% |
|
~% |
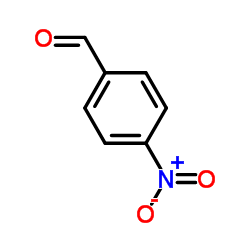
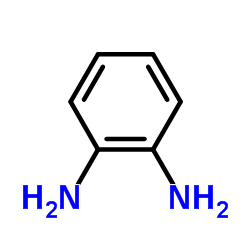
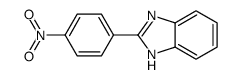
![2-(4-nitrophenyl)-1-[(4-nitrophenyl)methyl]benzimidazole结构式](https://image.chemsrc.com/caspic/074/54821-03-3.png)
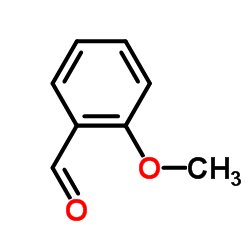
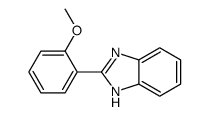
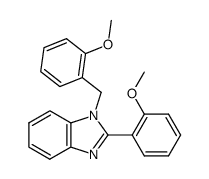
![N-[2-(benzylideneamino)phenyl]-1-phenylmethanimine结构式](https://image.chemsrc.com/caspic/315/15223-25-3.png)