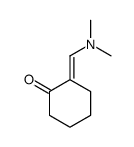
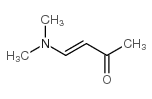
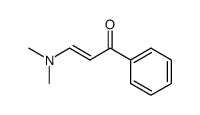
|
~31% |
|
~43% |
|
~72% |
|
~94% |
|
~34% |
|
~33% |
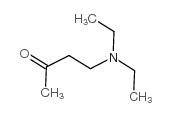
![Cyclohexanone, 2-[(dimethylamino)methyl]-, (+)- (9CI)结构式](https://image.chemsrc.com/caspic/281/769092-24-2.png)