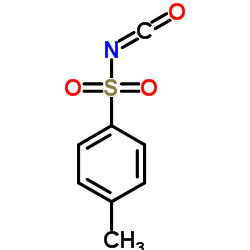
|
~% |
|
~89% |
|
~% |
|
~% |
|
~% |
|
~94% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~86% |
|
~% |
|
~% |
|
~% |
|
~% |
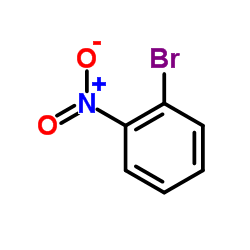
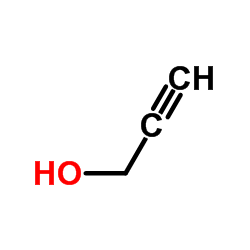
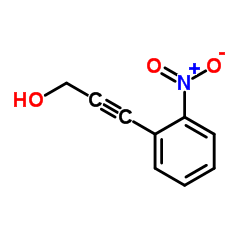

![4-methyl-N-[2-(2-phenylethynyl)phenyl]benzenesulfonamide结构式](https://image.chemsrc.com/caspic/142/442155-91-1.png)
![1-[1-(4-methylphenyl)sulfonyl-2-phenylindol-3-yl]ethanone结构式](https://image.chemsrc.com/caspic/040/62367-67-3.png)

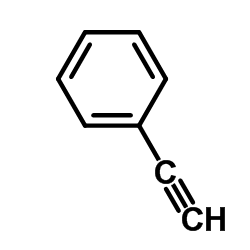
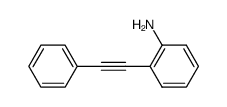


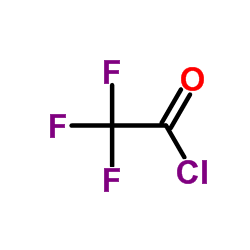
![2,2,2-trifluoro-N-[2-(2-phenylethynyl)phenyl]acetamide结构式](https://image.chemsrc.com/caspic/164/143360-89-8.png)