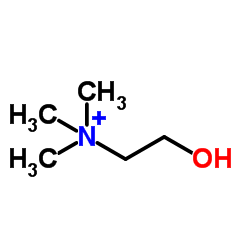
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
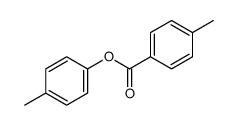

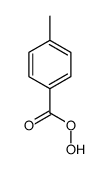

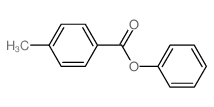

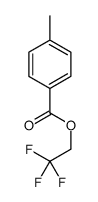
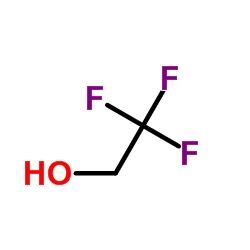
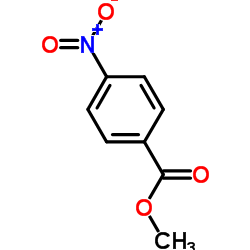
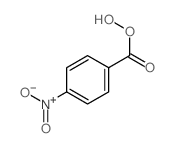

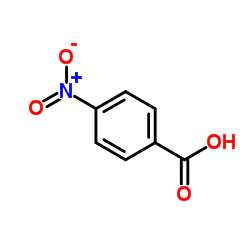
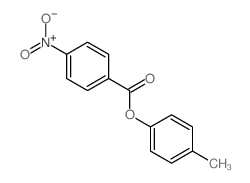
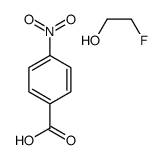
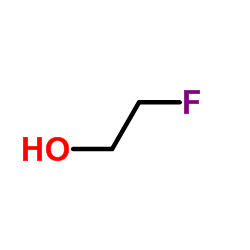

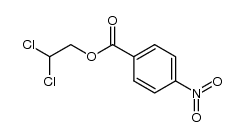
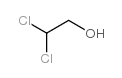
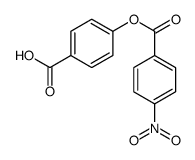
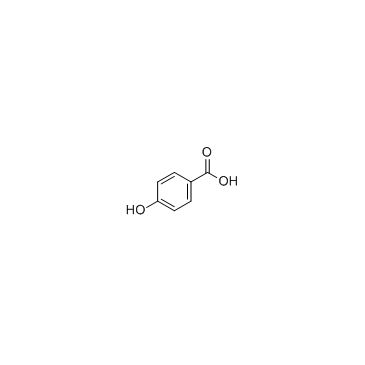
![trimethyl-[β-(4-nitro-benzoyloxy)-ethyl]-ammonium结构式](https://image.chemsrc.com/caspic/122/21105-92-0.png)