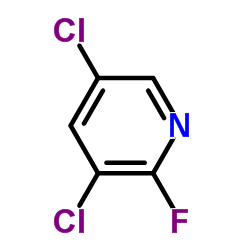
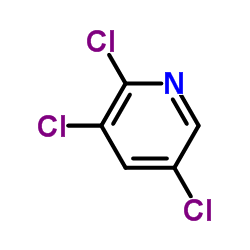
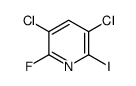
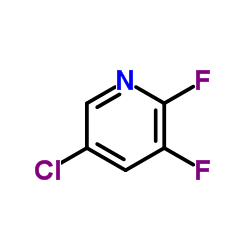
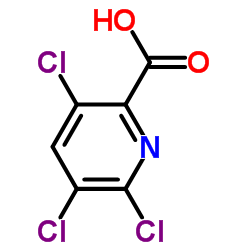
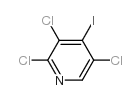
|
~70% |
|
~% |
|
~87% |
|
~55% |
|
~% |
|
~% |
|
~76% |
|
~77% |
|
~% |
|
~% |