|
~48% |
|
~54% |
|
~53% |
|
~55% |
|
~39% |
|
~38% |
|
~63% |
|
~37% |
|
~45% |
|
~94% |
|
~63% |
|
~41% |
|
~63% |

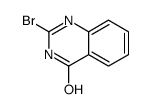
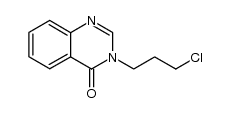

![3-[2-(2-bromo-1H-indol-3-yl)ethyl]quinazolin-4-one结构式](https://image.chemsrc.com/caspic/136/807354-69-4.png)
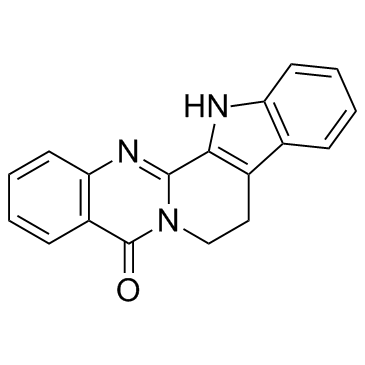
![3-[2-(1H-indol-3-yl)ethyl]quinazolin-4-one结构式](https://image.chemsrc.com/caspic/446/60941-86-8.png)
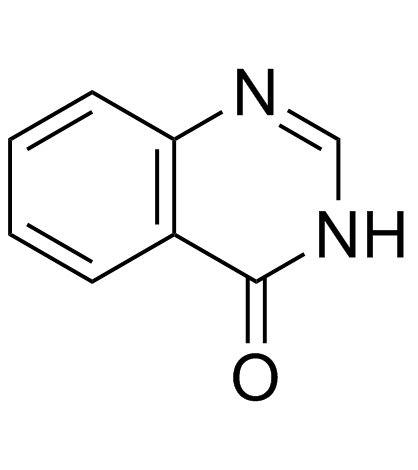

![3-[(2-iodophenyl)methyl]quinazolin-4-one结构式](https://image.chemsrc.com/caspic/413/923018-88-6.png)
![3-[2-(2-bromophenyl)ethyl]quinazolin-4-one结构式](https://image.chemsrc.com/caspic/375/923018-89-7.png)


![12H-isoindolo[1,2-b]quinazolin-10-one结构式](https://image.chemsrc.com/caspic/209/109439-60-3.png)