|
~% |
|
~% |
|
~% |
|
~% |
|
~85% |
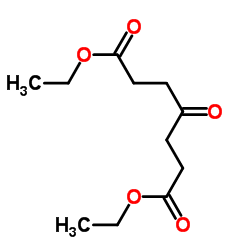
![ethyl 8-methyl-1,4-dioxaspiro[4.5]dec-7-ene-7-carboxylate结构式](https://image.chemsrc.com/caspic/307/76379-88-9.png)
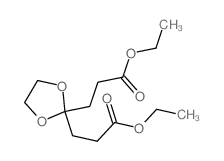
![ethyl 8-hydroxy-1,4-dioxaspiro[4.5]dec-7-ene-7-carboxylate结构式](https://image.chemsrc.com/caspic/400/121210-56-8.png)

![ethyl 8-((diethoxyphosphoryl)oxy)-1,4-dioxaspiro[4.5]dec-7-ene-7-carboxylate结构式](https://image.chemsrc.com/caspic/328/78385-47-4.png)
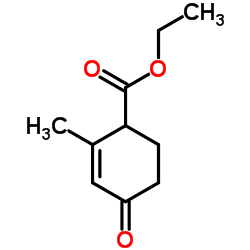
![7-甲基-1,4-二噁螺[4.5]-7-癸烯-8-羧酸乙酯结构式](https://image.chemsrc.com/caspic/239/32917-26-3.png)