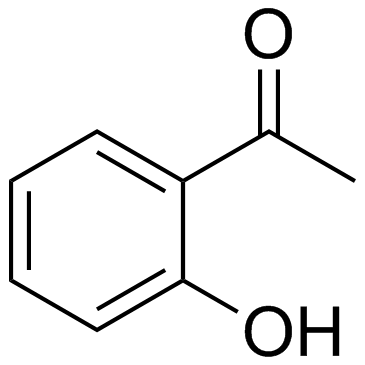
|
~94% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |



![4-(2-oxo-2H-[1]benzopyran)carboxaldehyde结构式](https://image.chemsrc.com/caspic/441/35893-95-9.png)