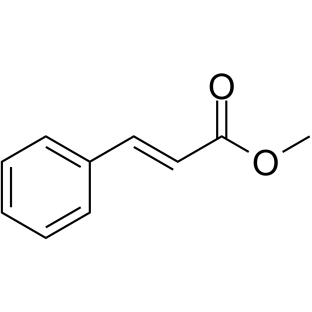
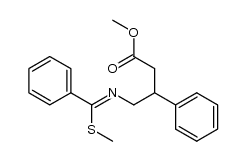
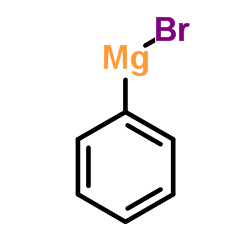
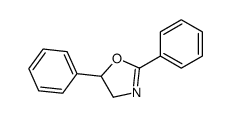
|
~14% |
|
~% |
|
~79% |
|
~% |
|
~62% |
|
~% |
![N-[1-(methylthio)benzylidene]-1-(trimethylsilyl)methylamine结构式](https://image.chemsrc.com/caspic/332/103738-73-4.png)