|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
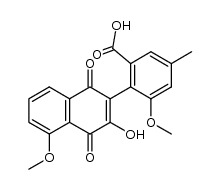
![8-hydroxy-1-methoxy-3-methylnaphtho[3,2-c]isochromene-5,7,12-trione结构式](https://image.chemsrc.com/caspic/182/76191-51-0.png)
![1,10-dimethoxy-8-methyl-11,12-dihydro-6H-dibenzo[c,h]chromene-6,11,12-trione结构式](https://image.chemsrc.com/caspic/232/281190-85-0.png)
![1,8-dimethoxy-3-methyl-7,12-dihydro-5H-dibenzo[c,g]chromene-5,7,12-trione结构式](https://image.chemsrc.com/caspic/179/122775-53-5.png)